The primary aim of this Milk SA project done by the UKZN was to identify bacteriophages that are specific to mastitis causing Staphylococcus aureus, and to test these phages in an in vivo setting against mastitis infections in dairy cows. Studies have shown that commonly used control measures such as antibiotics, cultural measures, culling, vaccines are now only partially effective in management of S. aureus induced mastitis. Thus, the primary problem identified is the development of antibiotic resistance in S. aureus. Because of the resistance, there is now no sustainable measure for the control of the disease. It is believed that bacteriophages (phages) could provide an alternative control measure worth looking into.
For the purpose of the laboratory trials, 272 raw milk samples were collected from two farms in the Mooi River district and the Allerton Provincial Veterinary laboratory. These were tested for the presence of S. aureus. More than 70% of the samples tested positive for S. aureus. The samples were subsequently also tested for antibiotic susceptibility or resistance.
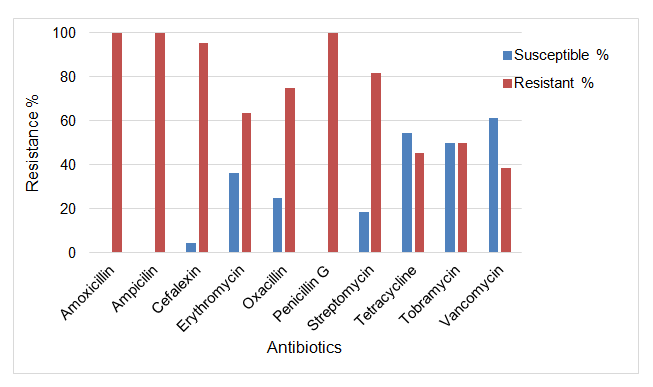
The results of antibiotic susceptibility testing of S. aureus isolates showed that 75-80% isolates were multidrug resistant. The isolates were found to be 100% resistant to amoxicillin, ampicillin and penicillin G, 95% resistant to cephalexin, 82% resistant to streptomycin, 75% resistant to oxacillin, 64% resistant to erythromycin, 50% resistant to tobramycin, 46% resistant to tetracycline and 39% resistant to vancomycin. None of the S. aureus isolates showed complete resistance to all antibiotics tested (Figure 1).
Figure 1. Antibiotic resistance patterns of S. aureus isolates against commonly used antibiotics on dairy farms in Republic of South Africa.
For the study on phage contol, the three phages, SaBp1, SaBp2 and SaBp3, previously shown by Dr Iona Basdew at UKZN to be highly potent in controlling S. aureus were used. These phages were used separately and then combined into a cocktail (phage cocktail). SaBp1, SaBp2 and SaBp3 showed very similar lytic activity against five S. aureus isolates tested. The phage cocktail exhibited much more lytic activity compared to the three phages alone. They also appeared to be highly virulent and produced higher plaque counts than SaBp1, SaBp2 and SaBp3 alone. The phage cocktail produced titres of 3.91x109 whereas SaBp1, SaBp2 and SaBp3 produced titres one log lower, respectively 2.19x108, 3.19x108 and 3.12x108pfu/ml.
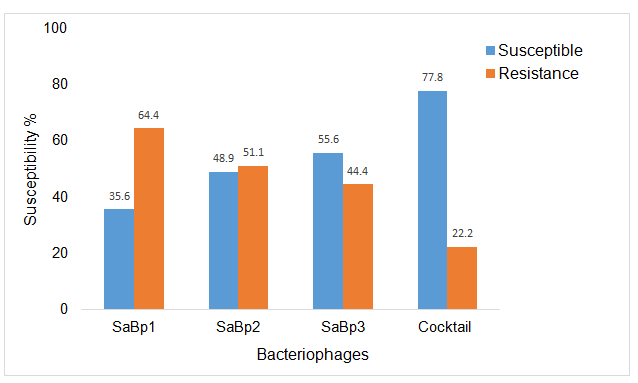
The S. aureus host range of strains of the three phages and the phage cocktail were tested for susceptibility against 44 S. aureus isolates obtained from raw milk samples from two dairy farms and the Allerton Provincial Veterinary Laboratory and one American type strain. SaBp1 showed the ability to produce zones of inhibition on 36% S. aureus isolates, SaBp2 49% and SaBp3 56%. The phage cocktail, which is the combination of the three phages, exhibited a surprising ability to produce zones of inhibition on 78% S. aureus isolates. These results (Figure 2) indicate that the phage cocktail has a very wide host range compared to the phages individually.
Figure 2. Host Range Assay showing susceptibility of 45 S. aureus isolates from three phages (SaBp1, SaBp2, and SaBp3) and phage cocktail.
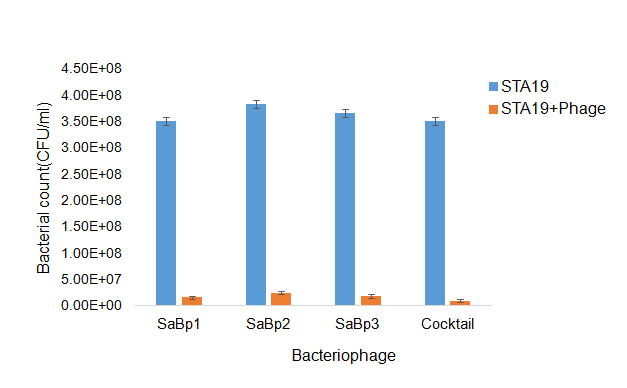
The so-called lethal dose assay was used to investigate phage efficacy in reducing live bacterial cell counts after incubation overnight. Phages SaBp1, SaBp2, SaBp3 and the phage cocktail were tested against S. aureus strain STA19. Highly significant differences in bacterial cell counts were found when STA19 was inoculated with phages in comparison to un-inoculated STA19. Bacterial cell counts decreased significantly upon introduction of phages into the bacterial culture (Figure 3). The phage cocktail showed greater reductions than the three phages applied independently. Phages SaBp1, SaBp2, SaBp3 reduced the live bacterial cell count by 93-95% and the cocktail reduced it by 97%.
Figure 3. Lethal dose assay showing S. aureus (STA19) growth with and without addition of phages and the effects of the phage cocktail compared, using each phage separate.
Finally, clinical in vivo trials were performed to assess the ability of the phage cocktail to cure pre-existing cases of S. aureus mastitis. Six cows were enrolled for the first trial, 10 for the second and 12 for the third. This resulted in 28 cows used for this experiment in two different locations. From each trial, the cows were half separated into two groups, phage cocktail-treated (treated) cows and 40% glycerol treated cows (untreated). The cows were milked twice daily at each site during each in vivo trial proceedings. The herd sizes ranged from 200 to 550 cows.
Phage treatment efficacy was evaluated based on the bacteriological cure in all cows enrolled in the study and by measurement of S. aureus shedding by treated udder quarter. Bacteriological cure was established by repeated post-treatment sampling and culture of milk collected from the treated quarter. Treated cows were sampled daily for one week during treatment and two weeks after treatment. Quarter milk samples were plated on the selective media for colony formation to calculate CFU/ml (S. aureus counts)and for the presence of plaques. Quarter milk samples collected were plated using the double-layer agar method on TSB supplement with bacteriological agar to calculate PFU/ml (phage counts) for both treated and untreated groups. The increase or decrease in CFU/ml was noted to determine if the S. aureus has been reduced or controlled within the treated group compared to the untreated group, and the presence of plagues within the treated and untreated group was noted to check the multiplication of the phage cocktail within the treated group.
Colony forming units (CFU/ml) and PFU/ml obtained from milk collected from treated and untreated cows were used to measure the S. aureus mastitis on treated and untreated cows. Bacteriological examination of these milk samples showed a daily reduction from the treated cows in the amount of S. aureus shed by quarters during the treatment trial and from the untreated cows. The amount of S. aureus was greater on the untreated cows compared to the treated cows; these results indicate that the use of the phage cocktail to treat S. aureus is effective in reducing mastitis caused by the organism. The results were the same for all three trials.
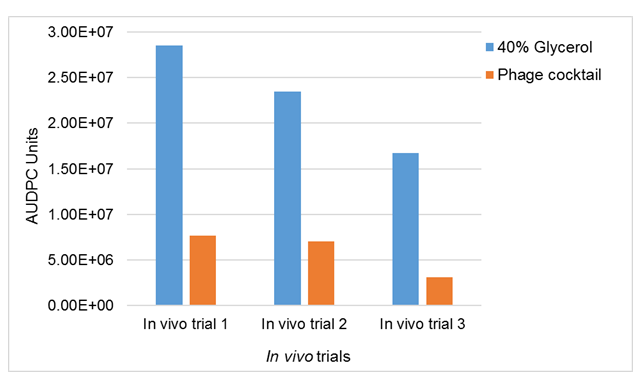
Figure 4 illustrates the difference or the effect of applying phage cocktail in cow teats that were infected with mastitis caused by S. aureus in the three in vivo trials. The results showed that mastitis was reduced by 73% in trial 1, by 70% in trial 2 and by 81% in trial 3. The results showed almost the same trend for all three trials. The reduction of S. aureus growth in milk samples collected from mastitic cows after phage cocktail treatment, showed that a phage cocktail can be used in dairy herds to reduce and control mastitis in dairy cows.
Figure 4. Effects of spraying of phage cocktail on the S. aureus mastitic lactating cows. Data are mean AUDPC of CFU/ml milk values from quarters which received 40-ml of spraying of phage cocktail (treated) (1.2 X 108) and 40% glycerol untreated. The data is for three in vivo trials.
In vivo trial 1: P-value= <.001, F-value= 2.92, CV%= 15.4; In vivo trial 2: P-value= <.001, F-value= 3.60, CV%= 3.0; In vivo trial 3: P-value= <.001, F-value= 3.06, CV%= 9.1.
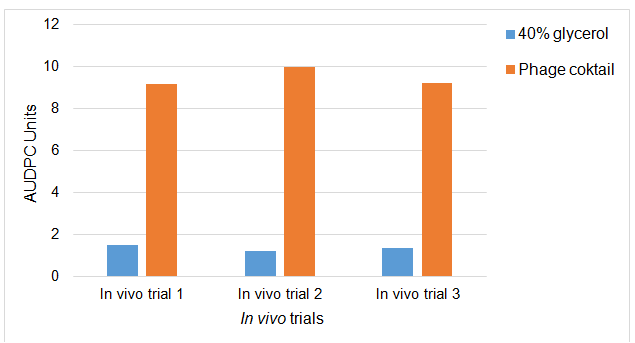
The AUDPC graph in Figure 5 shows the increase or multiplication of phage cocktail plaques after the application of the phage cocktail in mastitic dairy cow teats caused by S. aureus. As shown in the figure the application of phages increased and multiplied compared to the 40% glycerol treated cows. These results showed similar trends for all three trials. The results suggest that within the treated cows there were S. aureus and phages which could find the mastitis infected rear of the udder or teats, and they could colonise those rears and the phages could replicate and multiply after finding the S. aureus host. This indicates that the applied phages were viable and they can multiply and kill the bacterial host.
Figure 5. Increase of plaques population within milk samples collected from phage cocktail treated (1.2 X 108) and 40% glycerol (control) Staphylococcus aureus mastitic live dairy cows. Data are the mean AUDPC of log milk PFU/ml values from quarters which received 40-ml spraying of phage cocktail and 40% glycerol control. The data are for three in vivo trials.
In vivo trial 1: P-value= <.001, F-value= 4.22, CV%= 1.9; In vivo trial 2: P-value= <.001, F-value= 3.89, CV%= 3.9; In vivo trial 3: P-value= <.001, F-value= 4.28, CV%= 3.2.